Al3+ Ion Electron Configuration
Electron configuration al3 LIMITED TIME OFFER. Chemistry questions and answers.

Electron Configuration Of Al 3 Aluminum Ion Youtube
Which of the following is the electron configuration for the Al3 ion.

. The electron configuration of aluminum ion Al 3 is 1s 2 2s 2 2p 6. Well also look at why Aluminum forms a 3 ion and how the electron confi. The next six electrons will go in the 2p orbital.
This electron configuration shows that aluminum ion Al 3 has acquired the electron configuration of neon and it achieves an. When aluminum Al 13 reacts it loses its three valence electrons to achieve the same electron configuration as neon Ne 10 That configuration is. Shorthand Electron Configuration Full Electron Configuration Electron shell arrangement.
The two additional electrons required to fill the valence orbitals give the oxide ion the charge of 2 O 2. Aluminium is located in period 3 group 13 and has an atomic number equal to 13. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
Electronic configuration of Al 3. Al 3e Al 3. In this video we will write the electron configuration for Al 3 the Aluminum ion.
This tells you that the electron configuration of a neutral aluminium atom must account for a. Welcome to our channel K2chemistryclass This is an educational Channel specially for chemistryElectronic configuration full video-https. When the Aluminium Al atom loses three electrons it forms Al 3 ion.
Examples of isoelectronic species are N 3 O 2 F Ne Na Mg 2 and Al 3 all have the electron configuration 1s 2 2s 2 2p 6. The electron configuration for Aluminum is 1s2 2p2 3s6 3p1. Oxygen for example has the electron configuration 1s 2 2s 2 2p 4 whereas the oxygen anion has the electron configuration of the noble gas neon Ne 1s 2 2s 2 2p 6.
That is aluminum is a cation element. What is the ground-state electron configuration of the ion. Up to 256 cash back Get the detailed answer.
The p orbital can hold up to six electrons. Electron configuration of Helium He 1s 2. Electron Configuration for Fe Fe2 and Fe3 Iron and Iron Ions In writing the electron configuration for Iron the first two electrons will go in the 1s orbital.
The electronic configuration of Al 3 is 1 s 2 2 s 2 2 p 6. Your starting point here will be the electron configuration of a neutral aluminium atom Al. Electron configuration of Lithium Li He 2s 1.
Electron configuration of Beryllium Be He 2s 2. When determining the electron configuration of an ion for main group elements the electrons that were added last are the ones that are removed first when forming a cation. Predicting Electron Configurations of Ions.
1s 2 2s 2. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. Electron configuration of Hydrogen H 1s 1.
1s 2 2s 1. But for the Ion which is Al 3 it has a positive chargetherefore electrons need to be taken away not added also the octet rule must. So in the 3 oxidation state aluminium attain the noble gas electronic configuration.
What is the.

Al 3 Electron Configuration Aluminum Ion Youtube

Electronic Configuration Of Al Al3 Youtube

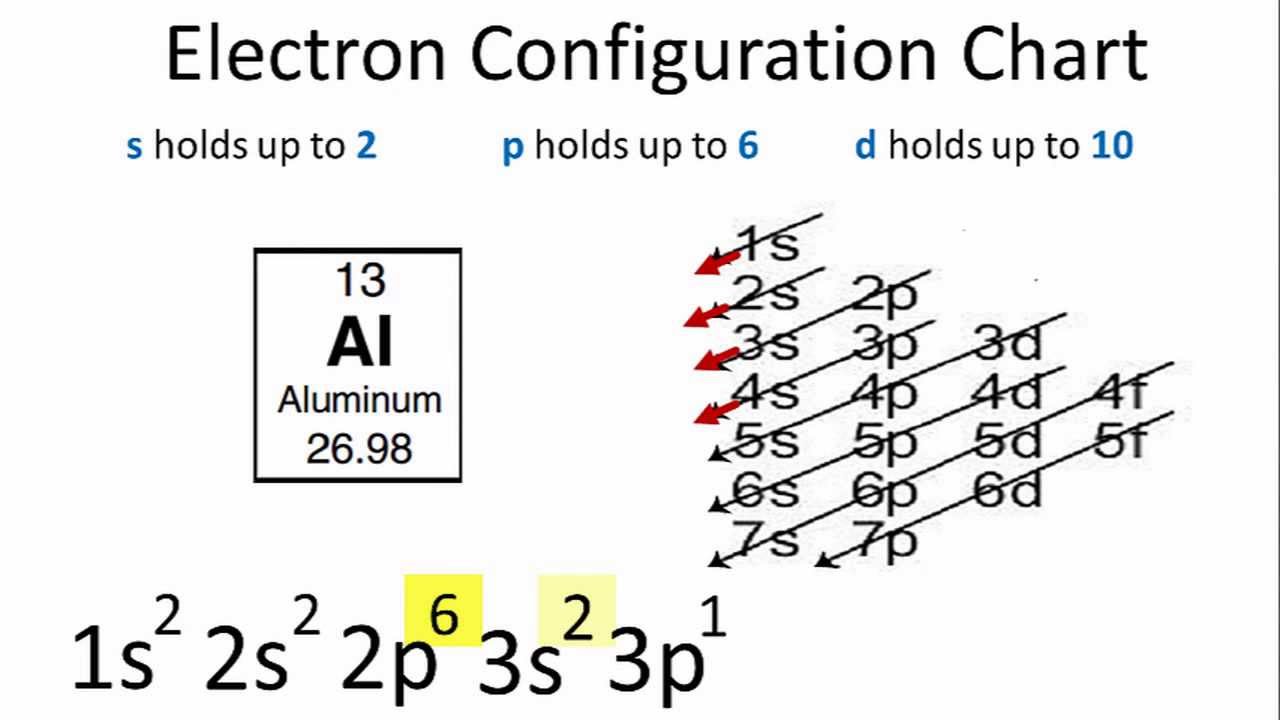
Aluminum Orbital Diagram Electron Configuration And Valence Electrons

Ba 2 Electron Configuration Barium Ion Youtube

Electron Configuration For Cu Cu And Cu2 Copper And Copper Ions Youtube
Comments
Post a Comment